Background and Objectives
Hereditary alpha-Tryptasemia (HaT) and Systemic Mastocytosis (SM) both display elevated basal serum tryptase (BST) levels and share mast cell-mediated digestive, neuropsychiatric, cardiovascular, cutaneous and musculoskeletal symptoms. Lack of awareness and numerous differential diagnoses complicate and often delay diagnosis, while patients suffer from impaired quality of life and do not receive suitable treatment. HaT is defined by an autosomal dominant inherited amplification of an alpha-Tryptase encoding TPSAB1 gene and is found in 5-6% of the general population. SM is characterized by an activating cKIT D816V mutation that can be detected in 90% of patients. Currently, there is no test available to discriminate between HaT and SM. We aimed to establish a non-invasive blood test supporting the diagnostic workup of these still unrecognized entities.
Methods
We established a customized digital droplet (dd)PCR triplex assay to detect HaT and a highly sensitive ddPCR singleplex assay for cKIT D816V mutation. We designed a clinical assessment form to record the symptoms. We identified the frequency of HaT and cKIT D816V mutation in genomic DNA isolated from peripheral blood buffy coat samples in 112 patients presenting to our institution with suspected mast cell-mediated symptoms and highly normal (>8-11.4ng/ml) or elevated (>11.4 ng/ml) BST levels (prevalence cohort, Fig. 1). For symptom assessment, we performed blood testing and symptom assessment in these 112 patients and 34 additional patients presenting with suspected SM (n=146, symptom cohort, Fig. 2). As a control cohort we tested 62 age- and sex-matched patients presenting with BST levels < 6 ng/ml (thus not suspected to have HaT or SM).
Results
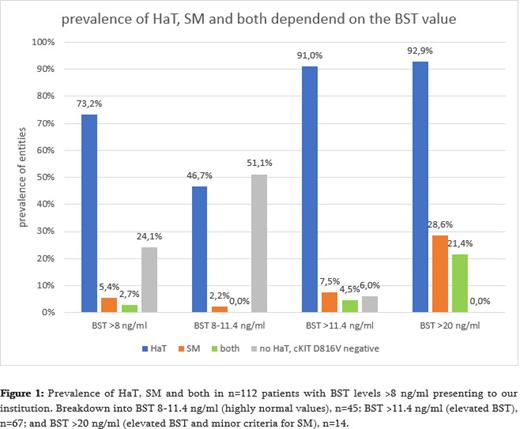
HaT accounted for 82 out of 112 cases (73.2%) of BST levels of 8 ng/ml or higher, while cKIT D816V indicating SM was identified in only 5 cases. In 28 patients (24,1%) no HaT genotype or cKIT D816V mutation was detected. One patient with negative cKIT D816V testing but clinical signs of SM was confirmed by bone marrow biopsy, increasing SM prevalence to 6/112 (5.4%). Interestingly the HaT and SM proportions increased to 91% (HaT) and 7.5% (SM) when higher BST cut-off level of 11.4 ng/ml and to 92,9% (HaT) and 28.6% (SM) when higher BST cut-off level of 20 ng/ml were used (Fig. 1). Of note, 21.4% of patients with BST > 20 ng/ml had both, HaT and SM, and no patient with BST >20 ng/ml was tested negative for HaT and SM. In the symptom cohort, we found significant differences in type and severity of symptoms between HaT, SM and the control group. The occurrence of digestive symptoms was up to twice as high in HaT or SM compared to the control group, for flatulence (HaT 83.5%; control group 53.2%) and diarrhea (SM 68.8%; control group 25.8%) or heartburn (SM 56.3%; control group 25.8%). Nausea was significantly more common in HaT (51.7%) than in SM patients (21.9%). HaT patients more frequently reported neuropsychiatric symptoms compared to control group and SM patients. This included memory impairment (HaT 45.9%; SM 23.3%; control group 16.1%) sleep disturbances (HaT 55.8%; SM 43.8%; control group 33.3%) and fatigue (HaT 67.4%; SM 50.0%; control group 37.7%).
Conclusions
Simultaneous, non-invasive testing for cKIT D816V and HaT in patients with suspected mast cell mediator symptoms allows discrimination of HaT from SM. Adding BST > 8 ng/ml and >11,4ng/ml to the diagnostic workup in symptomatic individuals increases the pretest probability to detect HaT to 73.8% and 91.0%. Diagnosed patients experience mental relieve through personal and medical acknowledgement of their symptoms and suitable treatment can be initiated. According to our results, HaT testing should be initiated in all individuals presenting with mast cell mediator symptoms and BST > 8 ng/ml, and cKIT testing should be initiated in patients with BST >11.4 ng/ml. In patients with BST >20 ng/ml, cKIT D816V positive blood testing or signs of advanced SM, bone marrow based diagnostic workup is still mandatory to cover diagnostic criteria for SM according to WHO.
Disclosures
Khandanpour:Janssen: Consultancy; Celgene: Consultancy; Astra Zeneca: Research Funding; Sanofi: Consultancy; Amgen: Consultancy; Kartos Therapeutics: Research Funding. Von Bubnoff:Takeda: Honoraria; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria; Novartis: Honoraria; Astra Zeneca: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal